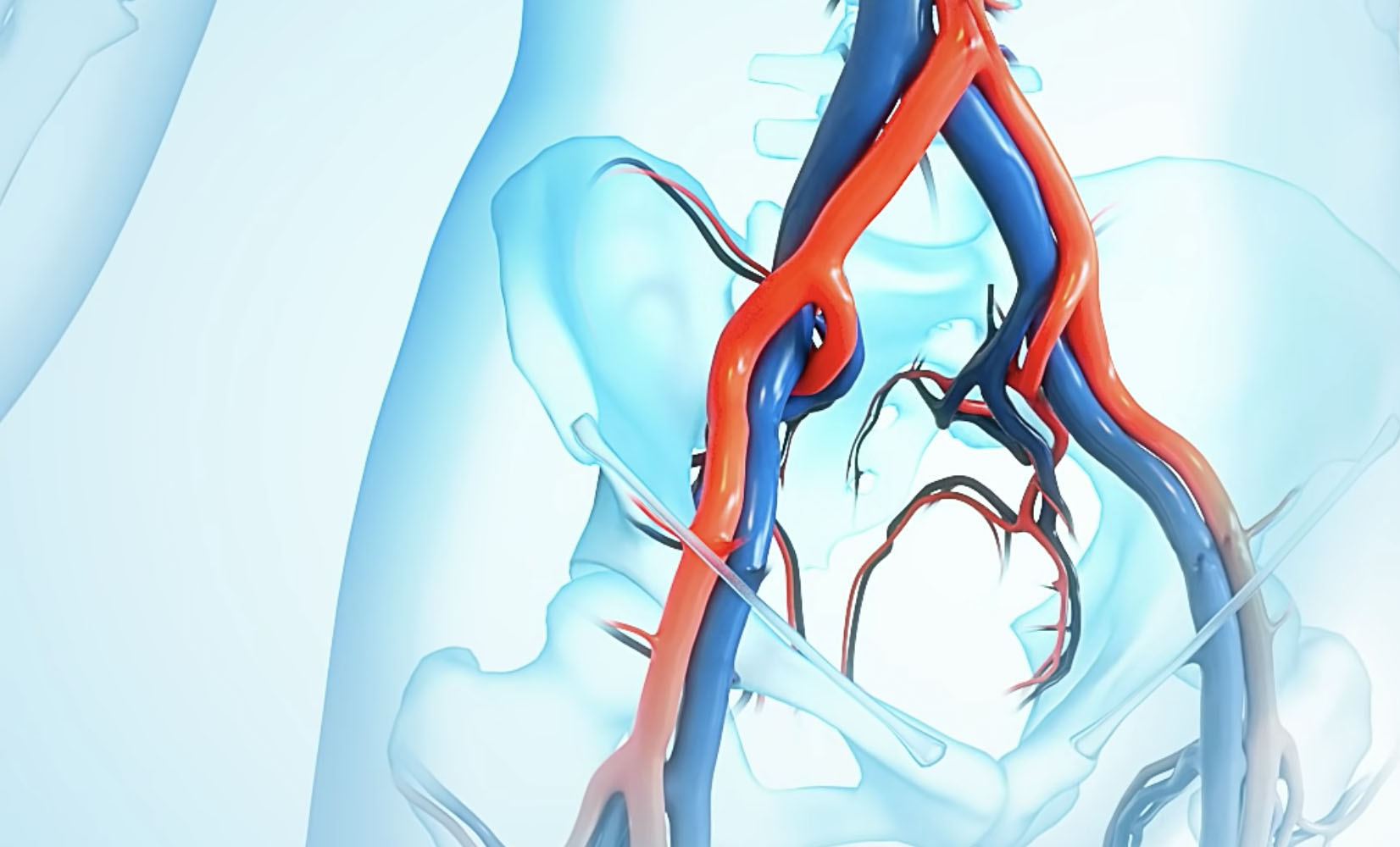
- Vesper Medical Announces Completion of Enrollment in the VIVID Clinical Trial
Company Achieves Key Milestone in the Development of its Next Generation Deep Venous Stent System Wayne, PA, December 20, 2021 –Vesper Medical, Inc., a developer of medical devices for deep venous disease, today announced the completion of enrollment in its pivotal study – Venous stent for the Iliofemoral Vein Investigational clinical trial using the Vesper - Read More >>
- Stacy Enxing Seng Joins the Vesper Medical Board of Directors
Wayne, PA, January 4, 2021 Vesper Medical, Inc., a developer of medical devices for deep venous disease, today announced that Stacy Enxing Seng has joined the board of directors effective immediately. An accomplished executive leader, Stacy has over 25 years in sales, marketing and operational leadership roles building innovative technology portfolios, demonstrated - Read More >>
- Vesper Medical Announces First Enrollment in the VIVID Trial
Malvern, PA, December 1, 2020 Vesper Medical, Inc., a developer of medical devices for deep venous disease, today announced initiation of its U.S. Food and Drug Administration (FDA) Investigational Exemption (IDE) study – Venous stent for the Iliofemoral Vein Investigational clinical trial using the Vesper DUO Venous Stent System® (VIVID). The VIVID Trial, which - Read More >>