Do you have narrowed or blocked veins in your legs causing you pain, swelling, skin discoloration or even ulcers?
The VIVID clinical trial is currently recruiting adult patients that have Chronic Venous Insufficiency (CVI) and are eligible for a venous stent in their iliac or common femoral veins.
Chronic Venous Insufficiency
Chronic venous insufficiency, or CVI, occurs when your leg veins don’t allow blood to return back to your heart. Normally, valves inside your deep leg veins keep blood moving against gravity and toward your heart. When the walls of your vein are weakened and valves are damaged, the blood cannot travel out of the legs. This leads to high pressure in the leg veins and as a result, a person with CVI can experience symptoms like severe swelling, pain, or even skin discoloration and ulcers.
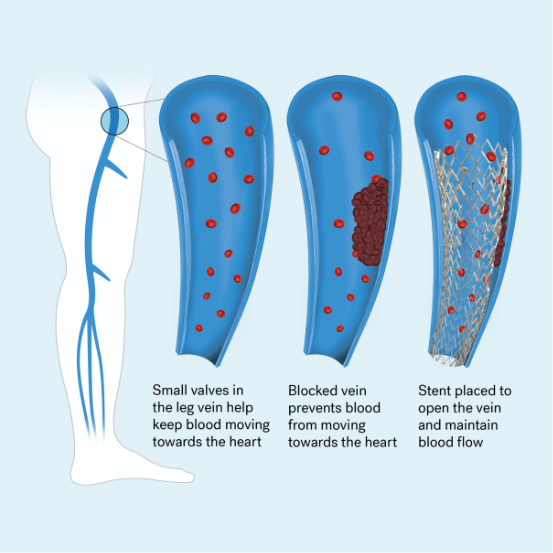
CVI can cause a vein in your leg to narrow or become completely blocked (occluded)
To treat blocked veins, a procedure called balloon venoplasty is performed. Venoplasty uses a tiny balloon catheter that is inserted in the blocked vessel to help widen it and improve blood flow. Venoplasty is often combined with the placement of a small metal tube called a stent. The stent helps keep the vein open, decreasing the chance of narrowing again.


Venous stent for Iliofemoral Vein Investigational clinical trial using Vesper DUO Venous Stent System©
The VIVID clinical trial is studying an investigational device called the Vesper DUO Venous Stent System that includes both the DUO-HYBRID™ and DUO-EXTEND™ stents. These new venous stents are intended to endure the unique forces and motion that happen in deep veins and are designed to reduce the symptoms associated with CVI. They are being studied to treat iliac and common femoral vein blockages with or without a history of a blood clot in the legs.
VIVID is a prospective, multi-center, single-arm, global IDE clinical trial evaluating the safety and efficacy of the Vesper DUO Venous Stent System in the treatment of iliofemoral occlusive disease.
Up to 160 subjects will be enrolled at up to 45 U.S. and International clinical sites.
This study is being led by principal investigator, Dr. Mahmood Razavi and is currently recruiting adult patients with CVI in the United States, Ireland, Poland and United Kingdom.
Speak with your doctor to see if you qualify to participate in the VIVID clinical trial.
Make sure to talk to your doctor about the potential benefits and risks associated with the Vesper DUO Venous Stent System, including other options for the treatment of your narrowed or blocked veins.

Contact us for information about Vesper Medical and the VIVID clinical study
* Required Field


