
Vesper Technology
Customize Therapy - Restore Venous Flow - Resolve Painful Symptoms
The Vesper DUO Venous Stent System® is designed to be the next generation venous stent portfolio uniquely engineered to address the multiple anatomical challenges of the deep venous system, providing physicians with a modular portfolio to customize therapy, restore venous flow, and resolve the painful symptoms of deep venous disease for the broad range of patients suffering from Chronic Venous Insufficiency (CVI).
Chronic venous insufficiency (CVI) is a common disease that affects nearly 30% of the adult population.1
One of the main causes of CVI is deep venous obstruction, which occurs when venous pressure increases and return of blood to the heart is impaired leading to multiple clinical complications in the lower extremities including: pain, swelling, edema, skin changes and development of venous ulcers.
Types of venous obstruction:
Thrombotic Obstruction2-5
-
Associated with acute deep vein thrombosis (DVT) or chronic post-thrombotic syndrome (PTS) due to a DVT
-
Acute DVT impacts more than 800,000 people annually in the US and can result in significant disability from pulmonary embolism and chronic venous insufficiency, especially when proximal iliofemoral veins are involved
-
Even with anticoagulation, PTS occurs in 50% of those with acute DVT and is the most frequent complication
Non-Thrombotic Obstruction, also known as non-thrombotic iliac vein lesion (NIVL)6
-
Caused by compression of the iliac vein by predominantly the left or right iliac artery and less frequently by the hypogastric artery
-
May-Thurner Syndrome (MTS) is the classic left-sided proximal lesion (compression) of the left iliac vein caused by the abrupt crossing of the right iliac artery
-
The right iliac artery may be related to proximal or distal NIVL of the right iliac vein, with the majority of lesions occurring near the right common iliac vein. Although less common, the left hypogastric artery crossing may also lead to left distal NIVL
-
90% of patients with significant symptoms of pain, swelling, skin changes, or venous stasis ulcers are found to have NIVL by IVUS examination7
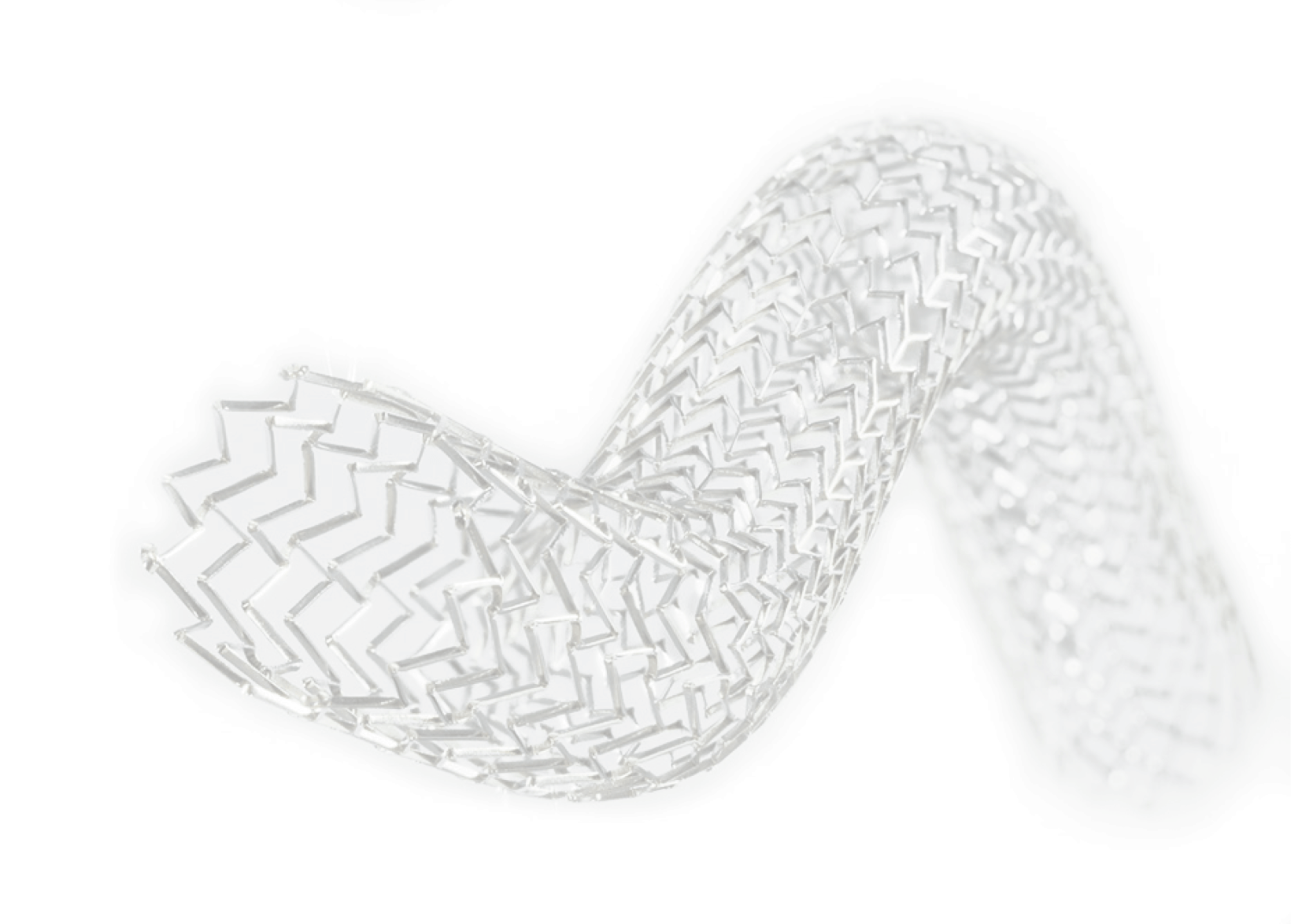
Deep Venous Stenting
Endovascular interventions with balloon dilation (venoplasty) and stenting of the iliac and common femoral veins have become first-line treatments of symptomatic deep venous outflow obstruction to maintain long-term patency, reduce patient symptoms, and improve quality of life.
Multiple indications with varying anatomical challenges
Thrombotic indications
Compressive indications
Tortuous anatomy
Varying diameters
Unique mechanical demands of deep venous stenting
High crush resistance in the common iliac vein
Flexibility and kink resistance coupled with crush resistance in pelvic region
High resistance to fracture around inguinal ligament
Prevention of edge crush to maintain patent inflow
A Portfolio for Deep Venous Disease
The Vesper DUO Venous Stent System consists of a portfolio of self-expanding venous stents (DUO-HYBRID™ and DUO-EXTEND™) intended to improve luminal diameter in symptomatic venous outflow obstructions. The portfolio approach includes delivery systems with either DUO-HYBRID or DUO-EXTEND venous stent, enabling the clinician to customize treatment in the iliofemoral venous anatomy based on disease patterns and severity.
Next Generation
Stent Design
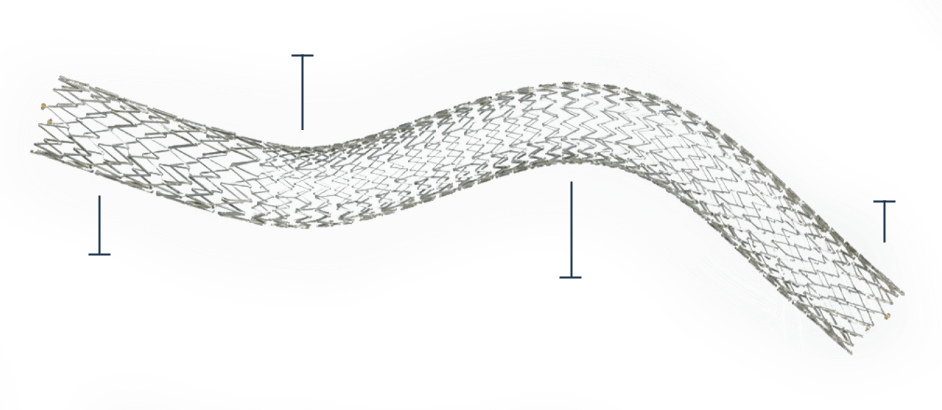
The DUO-HYBRID Stent
The DUO-HYBRID features a distinct integrated design that uniquely combines all necessary venous stent properties into a single stent.

Cranial strength paired with caudal flexibility to treat from IVC to common femoral vein
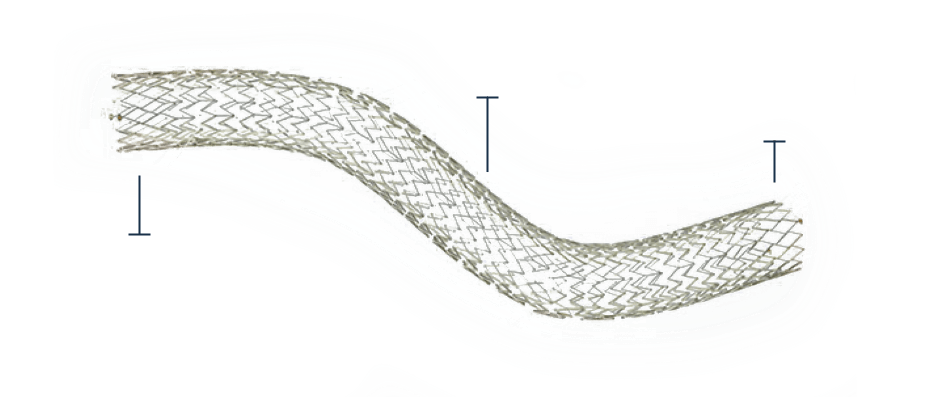
The DUO-EXTEND
The DUO-EXTEND is engineered to smoothly integrate with the DUO-HYBRID stent to extend therapy with superior durability, minimizing the risk of corrosion or fractures at the overlap point.

Flexibility to treat longer lesions
DUO-HYBRID and DUO-EXTEND stents are mounted on a familiar and high accuracy delivery platform. This, coupled with minimal stent foreshortening, ensures accurate deployment and full lesion coverage.

Customized Therapy for the Broad Range of Patients
The modular portfolio of both stent options offers the ability to treat the broad range of patients with chronic venous insufficiency and puts the control back in the physicians hands. From simple to complex lesions, the DUO-HYBRID and DUO-EXTEND stents are available in a full range of lengths and diameters providing the ability to customize therapy for each patient depending on the specific disease burden within the iliofemoral vein.
Key Design Features of the
DUO-HYBRID and DUO-EXTEND Stents
The DUO-HYBRID Stent
-
Self-expanding, open-cell nitinol stent features custom segments to address the challenging dynamics of deep vein mechanics
-
High crush resistant segment – provides crush resistance against outward radial force associated with May-Thurner area
-
Gradual transition segment transitions to a highly flexible segment designed to prevent foreshortening, fish scaling and optimize fatigue resistance for long-term durability in high strain areas such as the inguinal ligament
-
Inflow reinforcement located at the caudal end protects against edge crush, maintaining venous inflow
| Stent Type | Stent Diameter | Stent Lengths Available | Delivery System Size |
|---|---|---|---|
| DUO- HYBRID |
12mm 14mm |
60mm, 80mm, 100mm, 120mm, 140mm, 160mm | 9f |
| 16mm 18mm |
60mm, 80mm, 100mm, 120mm, 140mm, 160mm | 10f |
Flexible, kink-resistance in the pelvic and inguinal zones
Reduced edge crush to maintain inflow
High crush resistance in the MTS zone
Minimal foreshortening, fish scaling and gap formation
The DUO-EXTEND Stent
-
Self-expanding, open-cell nitinol stent designed to integrate with the DUO-HYBRID stent to extend treatment
-
Highly flexible segment to mitigate kinks during deep knee and hip flexion
-
Inflow reinforcement to mitigate end crush of the stent
-
Offers a modular taper for long lesions when the vein diameter changes
| Stent Type | Stent Diameter | Stent Lengths Available | Delivery System Size |
|---|---|---|---|
| DUO- EXTEND |
12mm 14mm |
40mm, 80mm, 100mm, 120mm, 140mm | 9f |
| 16mm | 40mm, 80mm, 100mm, 120mm, 140mm | 10f |
Overlap Segment
Flexible, kink-resistance with minimal foreshortening, fish scaling and gap formation
Reduced edge crush to maintain inflow
Safety Information
Intended Use:
-
The Vesper DUO Venous Stent System is intended for use in the iliac and common femoral veins for improving luminal diameter in symptomatic venous outflow obstructions
Contraindications for Use:
-
Patients with a known hypersensitivity to nickel-titanium alloy (Nitinol)
-
Patients unable to receive standard medication used for interventional procedures including anticoagulants, contrast agents and antiplatelet therapy
-
Patients who are judged to have a lesion that prevents complete inflation of a balloon dilation catheter or proper placement of the stent or the stent delivery system
-
Tortuous vascular anatomy significant enough to prevent safe introduction and passage of the device
-
The Vesper DUO stent is not designed for use in procedures that require jugular or contralateral access and therefore is absolutely contraindicated from these access sites
MRI SAFETY INFORMATION
Non-clinical testing has demonstrated that the DUO-HYBRID and DUO-EXTEND Venous Stents are MR Conditional when used per the intended use for all clinically relevant lengths. A patient with this device can be safely scanned in an MR system meeting the following conditions:
-
Static magnetic field of 1.5 T or 3 T, only
-
Maximum spatial gradient magnetic field of 4,000-gauss/cm (40-T/m)
-
Maximum MR system reported, whole body averaged specific absorption rate (SAR) of2-W/kg (Normal Operating Mode)
-
Circularly polarized (quadrature-driven) coil only
Under the scan conditions defined, an implant from the Vesper DUO Venous Stent System is expected to produce a maximum temperature rise of less than 2.0°C after 15-minutes of continuous scanning (i.e., per pulse sequence).
In non-clinical testing, the image artifact caused by the device extends approximately 5-mm from the Vesper DUO Venous Stent System when imaged with a gradient echo pulse sequence and a 3-T MR system. The lumen of this stent could be visualized on the T1-weighted, spin echo and gradient echo MR images.
References
-
Yost ML. Chronic venous disease (CVD) epidemiology, costs and consequences. Beaufort (SC): THE SAGE GROUP; 2016.
-
Lichtenberg M, de Graaf R, Erbel C. Standards for recanalisation of chronic venous outflow obstructions. Vasa. 2018 Jun;47(4):259-266.
-
Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011 Feb;86(2):217-20.
-
Thukral S, Vedantham S. Catheter-based therapies and other management strategies for deep vein thrombosis and post-thrombotic syndrome. J Clin Med. 2020 May 12;9(5):1439.
-
Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019 Feb 26;139(9):1162-1173.
-
Raju, S and Neglen, P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: A permissive role in pathogenicity. J Vasc Surg. 2006; 44:136-44.
-
Raju, S. Phlebolymphology 2008 15(1):12-16.

Contact us for information about Vesper Medical and the VIVID clinical study
* Required Field
